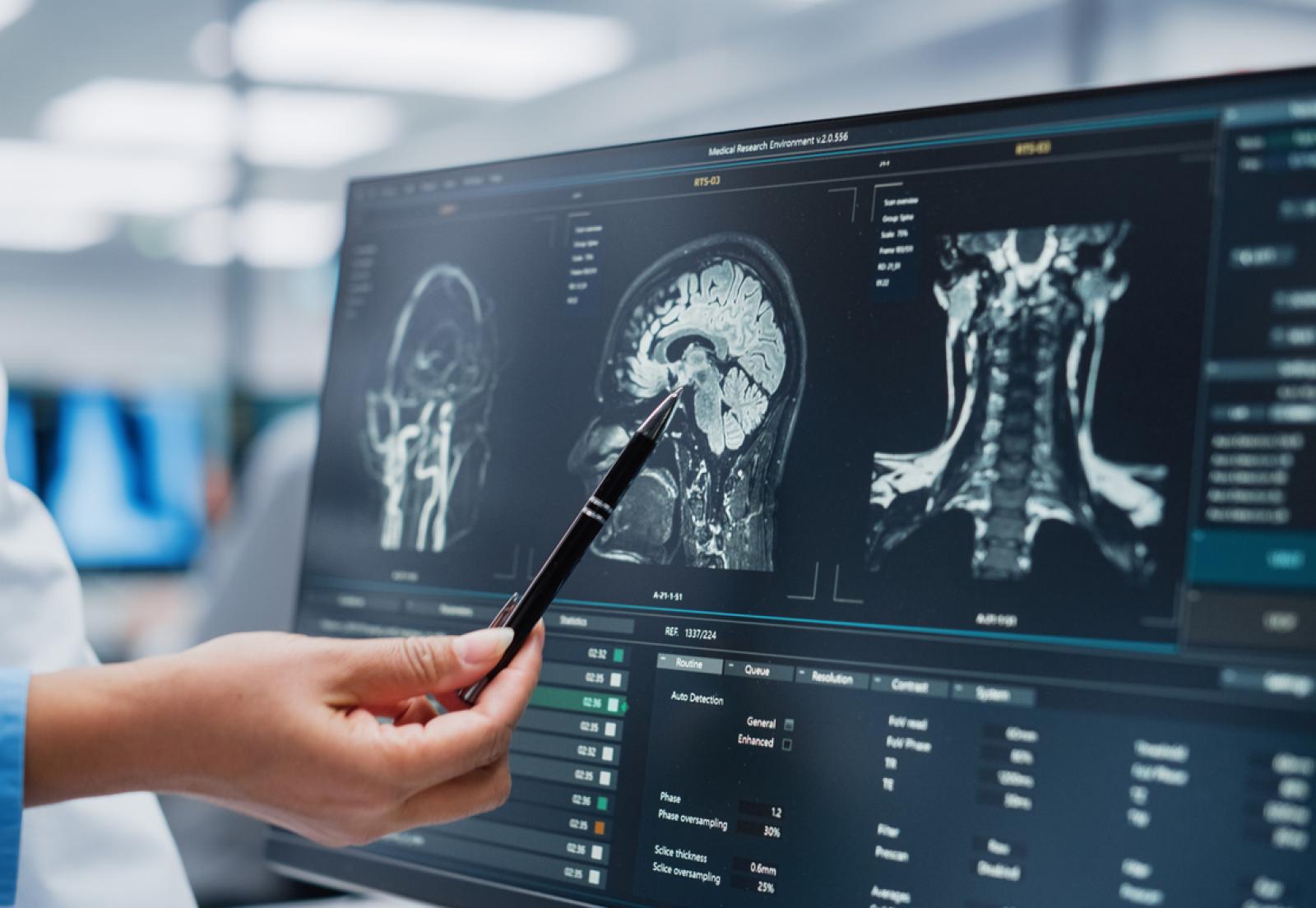
AI’s Role in Diagnosis
Diagnosis may sound leaps and bounds more complicated than simple predictive analytics, but at its core there’s not much of a fundamental difference. While we’re not close to replacing doctors with friendly robots, the models are essentially doing what seasoned practitioners do: recalling information about a subject, comparing data, scans and charts, and making assumptions about them. This can be monumental for expedience and limiting invasive diagnostic procedures. One example is the case of a woman with a thyroid lump that won’t go away. Upon examination of an ultrasound, her doctor ordered a biopsy of the lump (which turned out to be benign).
She then went to another radiologist for a second opinion, who came to the same conclusion. The second radiologist, however, used AI-driven thyroid ultrasounds to make their determination. Had she started here, an invasive test with weeks of waiting for results could have been prevented. In another instance, Penn Medicine researchers developed a tool that is capable of detecting cancer cells that are easy to miss, or even invisible, to the eye. Beyond sheer precision, it’s able to analyze and reconstruct enormous amounts of data in a very short time.
Perhaps the most promising fact is that AI can be trained to scan imaging like MRIs to identify and flag potential tumor-like structures in patients’ scans with incredible efficiency. This can help radiologists and oncologists who can do a deeper examination of these flagged areas. It may be a while before these tools become ubiquitous in healthcare settings, but it’s clear that there’s an emerging use case for machine learning to aid in the things that humans can’t do efficiently, quickly, or sometimes at all.
Early detection in cancer
“Outcomes can be completely transformed – better survival and less invasive treatments – if the cancer is diagnosed early enough, but we’re just hopeless at doing that. We need to find a way to do it better.” (University of Cambridge)
The use of AI in Radiology
Cancer poses a serious threat to human health worldwide and is a leading cause of death. The analysis of radiological imaging is crucial in early detection, accurate diagnosis, effective treatment planning, and ongoing monitoring of patients with cancer. With the development of computing resources, AI technologies have been widely applied in the field of radiological cancer imaging. Numerous AI models, through continuous refinement, have achieved performance comparable to or even surpassing that of radiologists in identifying various types of lesions. In recent years, AI has been effectively used in the detection of pulmonary nodules, breast cancer, and colon cancer. These successful applications have prompted the evaluation of AI approaches in more complex decision-making tasks, including cancer diagnosis, predicting treatment responses, and assessing disease prognosis. Herein, we compare mainstream AI methods used in the field of radiology and illustrate their applications in tumour imaging analysis. We also discuss the current limitations of these AI methods and explore potential directions for future AI advancements, to better integrate AI into clinical practice.
Personalised Cancer Treatment Strategies
One of the greatest challenges in cancer treatment is maximising the impact for the patient from drug treatments. Conventional treatment strategies, which focus on killing as many cells as possible, are based on a ‘maximum tolerated dose’ (MTD) therapy, where the patient continually receives a high drug dose, with no breaks in treatment. However, these frequently fail against metastatic cancers due to the emergence of drug resistance.
Adaptive therapy strategies, which dynamically adjust treatment to suppress the growth of treatment-resistant populations, have emerged as a promising alternative. However, the lack of personalized approaches that account for patient variation limits their efficacy.
(University of Oxford Study)
AI’s Role in Treatment
AI has emerged as a practical tool in cancer intervention and treatment in a variety of ways. We’ve already seen similar applications in this realm as we have with treatment and prevention. For example, a 2023 study showed that AI can have a crucial impact on precision medicine and the development of treatment plans. It did this by assisting in the prediction of treatment effects for tumour patients, personalizing treatment based on the unique cases of the patients, and much more as a result of mining “the deep-level information in genomics”. But it’s not all data-based; AI is becoming hands-on. It’s shown potential to optimize radiation doses, assist in surgical procedures, and offer on-the-fly adjustments to treatment plans that often need them.
Further, its role in research cannot be understated. It’s not easy or cheap researching, developing, and bringing to market a new treatment or drug, however promising it may be. AI has the potential to not only streamline these processes but make discovery and development more efficient than ever. One example of this is AlphaFolds2, which, with breakthroughs in understanding protein structure and more, is said to enhance the speed and precision of drug target identification.
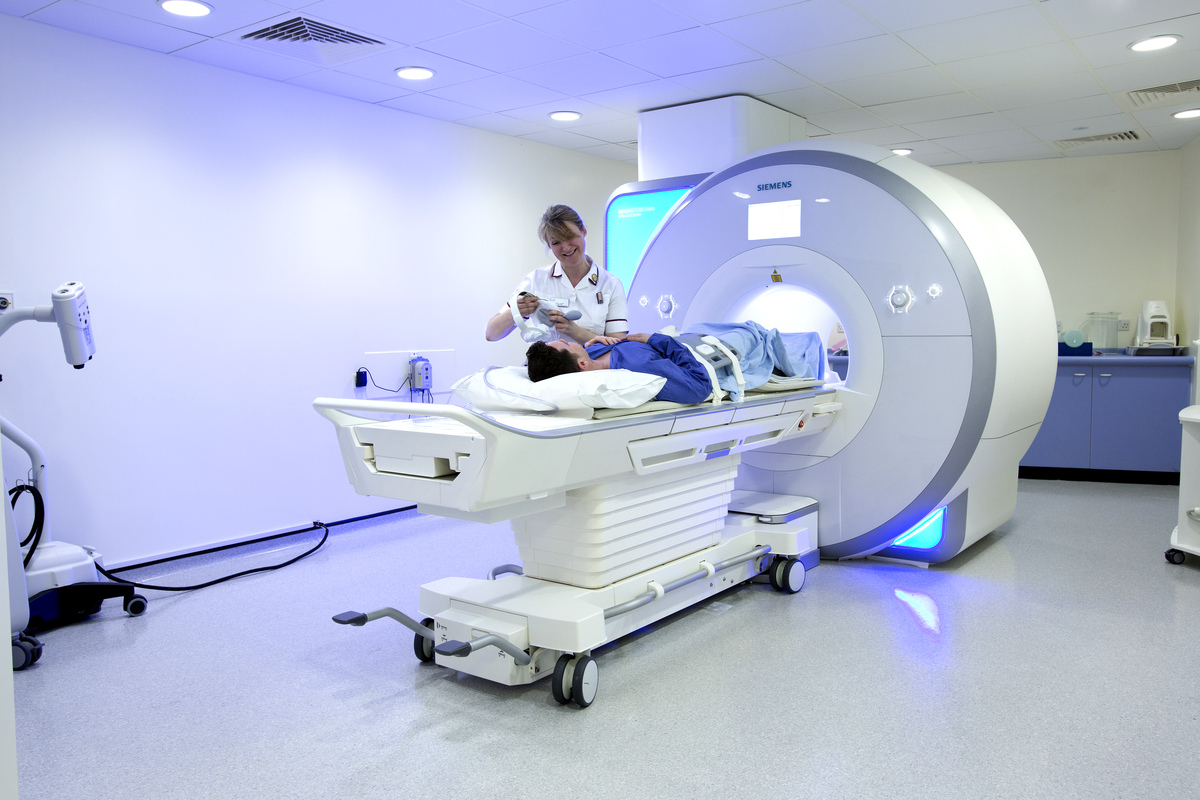
Expediting cancer screening, detection, and diagnosis
AI is helping to improve the speed, accuracy, and reliability of some cancer screening and detection methods. The Food and Drug Administration has authorized the marketing of AI-based software to help pathologists identify areas of prostate biopsy images that may contain cancer. Medical images such as mammograms can be rapidly processed with the help of AI, allowing radiologists to focus their time on other tasks that require their technical judgement. NCI-supported research has shown that AI imaging algorithms not only improve breast cancer detection on mammography but can also help predict long-term risk of invasive breast cancers. NCI scientists are using AI to improve cervical and prostate cancer screening. One group of NCI researchers and their collaborators developed a deep learning approach for the automated detection of precancerous cervical lesions from digital images.
Improving cancer surveillance
Cancer surveillance is the ongoing collection of patient information and cancer statistics. AI methods are being used to accelerate information extraction for surveillance reporting and to identify patterns in population-level cancer data. For example, a collaboration between NCI and the Department of Energy called Modelling is using AI approaches to submit data to NCI’s Surveillance, Epidemiology, and End Results (SEER) program more quickly. As part of this effort, scientists developed AI algorithms to extract tumor features automatically from unstructured clinical text, saving thousands of hours of manual processing time. This process will help researchers better understand how new diagnostic methods, treatments, and other factors affect patient outcomes.
- NCI-supported researchers are developing deep learning algorithms trained on population-level disease data to predict a person’s risk of pancreatic cancer, paving the way for early detection.
- Large language models for electronic health record surveillance. Exit Disclaimer are helping researchers better understand social determinants of health that may be critical for preventing, detecting, and treating cancer.
Improving access to cancer care
AI tools could also help make high-quality care accessible to more patients, even those who live far from cancer specialists or in low-resource settings, potentially helping to reduce cancer health disparities. With the emergence of chatbots, cancer researchers are probing whether this technology can be used in cancer care. Some studies have suggested that chatbots could help provide patients with tailored cancer information and even help draft physician responses to patient questions.
Early cancer detection - Survival rates increase to 80%
AI to improve cancer imaging
Artificial intelligence gives cancer research a boost
How AI predicts cancer 6 years in advance (2min)
Osairis AI clip 02
Artificial Intelligence New Research Teams (AI) Pancreatic Cancer Collective
Working With What We Already Have
While we don’t have all the answers yet, there’s no shortage of scientific literature on the disease. Decades of clinical trials, studies, papers, and more have left us with a seemingly infinite number of data points and takeaways.
The trouble is humans aren’t computers. No one person can find, absorb, and recall every relevant piece of information available at any given time. Large Language Models (LLM) can, though.
AI creates immeasurable efficiency when it comes to aggregating, recalling, and contextualizing complicated datasets without the element of human error. In other words, with a few short prompts, advanced models can:
1. Process irrationally large amounts of data
2. Identify patterns
3. Make predictions
4. Perform analysis
that researchers would otherwise need to sleuth manually. Science, being fundamentally iterative in nature, can make better use of things we already know to progress, and cancer research is no exception.
Perhaps even more notably, the future potential for this technology to streamline studies, analysis, trial design, and recruitment may well create an exponential impact on our race to the finish line for a cure.
Types of Cancers
- Lung Cancer: Cancer that develops in the lungs.
- Colorectal Cancer: Cancer of the colon or rectum.
- Prostate Cancer: Cancer that develops in the prostate gland (in men).
- Breast Cancer: Cancer that forms in the breast tissue.
- Skin Cancer: A broad category including melanoma and non-melanoma skin cancers.
- Bladder Cancer: Cancer that starts in the bladder.
- Kidney Cancer: Cancer that originates in the kidneys.
- Liver Cancer: Cancer that develops in the liver.
- Ovarian Cancer: Cancer that develops in the ovaries.
- Pancreatic Cancer: Cancer that begins in the pancreas.
- Stomach Cancer: Cancer that forms in the stomach.
- Uterine Cancer: Cancer that develops in the uterus.
The Expanded use of AI in Radiology
Cancer poses a serious threat to human health worldwide and is a leading cause of death. The analysis of radiological imaging is crucial in early detection, accurate diagnosis, effective treatment planning, and ongoing monitoring of patients with cancer.
With the development of computing resources, AI technologies have been widely applied in the field of radiological cancer imaging. Numerous AI models, through continuous refinement, have achieved performance comparable to or even surpassing that of radiologists in identifying various types of lesions. In recent years, AI has been effectively used in the detection of pulmonary nodules, breast cancer, and colon cancer. These successful applications have prompted the evaluation of AI approaches in more complex decision-making tasks, including cancer diagnosis, predicting treatment responses, and assessing disease prognosis. Herein, we compare mainstream AI methods used in the field of radiology and illustrate their applications in tumour imaging analysis. We also discuss the current limitations of these AI methods and explore potential directions for future AI advancements, to better integrate AI into clinical practice.
AI’s Role in Prevention of Cancer
Prevention and early detection are the most powerful weapons we have against cancer. But prevention is easier said than done, so to speak. With the vast number of contributing causes of cancer, and a radical variance in when and how early warning signs present, it can be hard to know when screening is necessary. Beyond that, access to screening remains a challenge, whether due to proximity to specialists, socioeconomic barriers, medical literacy, or something else. AI is making breakthroughs that could remove all these obstacles and more, and revolutionize prevention and early detection.
In 2023, research was conducted to explore how AI could aid in predicting incidences of pancreatic cancer, which is traditionally more difficult and expensive to screen for compared to other cancers. Using disease codes and their timing of occurrence from millions of patient records, the model predicted which patients were at highest risk for developing pancreatic cancer, despite the fact that “many of the symptoms and disease codes were not directly related to or stemming from the pancreas.”
The model was not only more accurate than population estimates but was also believed to be “at least as accurate in predicting disease occurrence as are current genetic sequencing tests that are usually available only for a small subset of patients in data sets. In short, this algorithm accomplished something that usually requires difficult-to-access genetic testing using millions of data points, many of which may have been entirely overlooked by a human. At scale, the possibilities are endless, and advanced models could very well aid the future of early detection by finding trends and relations between health, environmental, and behavioural factors that cause cancer.
AI tool predicts response to cancer therapy
Chemotherapy, radiation, and surgical removal of tumours have long been the standard approaches for treating different types of cancer. But in recent decades, different immunotherapies have become available. These rely on the body’s immune system to find and destroy cancer cells. One type of immunotherapy, called checkpoint inhibition, has greatly improved the treatment of many types of cancer. Immune checkpoint inhibiting drugs can make cancer cells more vulnerable to immune system attack. But they don’t work for everyone.
Scientists have been looking for better ways to identify patients most likely to respond to these drugs. People who probably wouldn’t benefit from them could avoid unnecessary treatments and side effects and be given different treatments. To date, two biomarkers have been approved by the U.S. Food and Drug Administration to identify patients most likely to benefit from these medications. One measures tumour mutational burden, which is the number of DNA mutations in cancer cells. But results of these tests are not always accurate. Other predictive tests depend on tumour molecular data that are costly to obtain and not routinely collected.
Acquired gene mutations and cancer
An acquired gene mutation is not inherited from a parent. Instead, it develops at some point during a person’s life. Acquired mutations occur in one cell and then are passed on to any new cells that come from that cell. This mutation cannot be passed on to a person’s children, because it doesn’t affect their sperm or egg cells. This type of mutation is also called a sporadic mutation or a somatic mutation.
Acquired mutations can happen for different reasons. Sometimes they happen when a cell’s DNA is damaged, such as after being exposed to radiation or certain chemicals. But often these mutations occur randomly, without having an outside cause. For example, during the complex process when a cell divides to make 2 new cells, the cell must make another copy of all of its DNA, and sometimes mistakes (mutations) occur while this is happening. Every time a cell divides is another chance for gene mutations to occur. The number of mutations in our cells can build up over time, which is why we have a higher risk of cancer as we get older. Acquired gene mutations are a much more common cause of cancer than inherited mutations.
Oxford University Launches Major New AI Cancer Vaccine Research Programme With The Ellison Institute of Technology (EIT)
The University of Oxford through it’s strategic partnership with the Ellison Institute of Technology (EIT) has received research funding of £118m to launch an ambitious new programme of vaccine research. Led by the Oxford Vaccine Group, the new initiative based in the University – CoI-AI (Correlates of Immunity-Artificial Intelligence) – will combine Oxford’s expertise in human challenge studies, immune science and vaccine development with EITs cutting edge Artificial Intelligence (AI) innovation technology to better understand how the body fights infection and how vaccines protect us.
The CoI-AI programme will study how the immune system responds to important germs that cause serious infections and contribute to antibiotic resistance – such as Streptococcus pneumoniae, Staphylococcus aureus, and E. coli, – amongst others, which cause widespread illness but have resisted traditional vaccine approaches. Researchers will use human challenge models (where volunteers are safely exposed to bacteria under controlled conditions) and apply modern immunology and AI tools to pinpoint the immune responses that predict protection. Professor Sir Andrew Pollard, Director of the Oxford Vaccine Group, said: ‘This programme addresses one of the most urgent problems in infectious disease by helping us to understand immunity more deeply to develop innovative vaccines against deadly diseases that have so far evaded our attempts at prevention. By combining advanced immunology with artificial intelligence, and using human challenge models to study diseases, CoI-AI will provide the tools we need to tackle serious infections and reduce the growing threat of antibiotic resistance. This is a new frontier in vaccine science.’
Professor Daniela Ferreira, Deputy Director of the Oxford Vaccine Group, said: ‘This programme will give us completely new tools to study how vaccines work at both a cellular and system-wide level, by studying infections in real time, in people, and using smart immunology tools and data to find the answers. This will open up whole new avenues to vaccine design as we improve our understanding of infection and immunity”.
